
Admission open for the Session 2026-2027 – Register Now!
2nd Law Of Thermodynamics
2nd Law Of Thermodynamics, Entropy, Heat Transfer
Dr. Nilkamal Barai
12/26/20241 min read
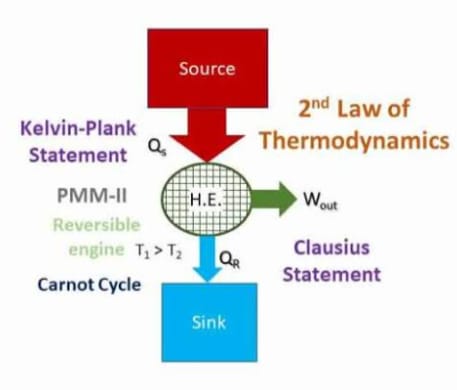
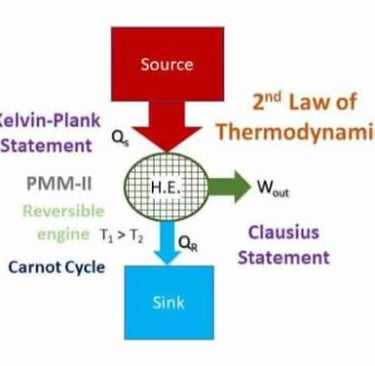
The Second Law of Thermodynamics is a fundamental principle of physics that describes the natural tendency of energy systems. It has several equivalent statements, but the core idea is that energy spontaneously disperses and systems naturally progress toward states of greater disorder (entropy).
Key Statements of the Second Law:
1. Entropy Increase: In any natural thermodynamic process, the total entropy of an isolated system always increases or remains constant. It never decreases.
\Delta S_{\text{total}} \geq 0
2. Heat Transfer: Heat energy cannot spontaneously flow from a colder body to a hotter body without external work being performed.
3. Efficiency Limits: No heat engine operating in a cycle can convert all absorbed heat into work. There is always some waste heat released to the surroundings.
4. Directionality: Natural processes are irreversible and have a preferred direction (e.g., heat flows from hot to cold).
Practical Implications:
It explains why perpetual motion machines (of the second kind) are impossible.
It governs the efficiency of engines, refrigerators, and other thermodynamic systems.
It underpins the arrow of time, as it links entropy with the progression of events.
In essence, the Second Law highlights the inevitability of energy spreading out and the tendency toward disorder in the universe.
